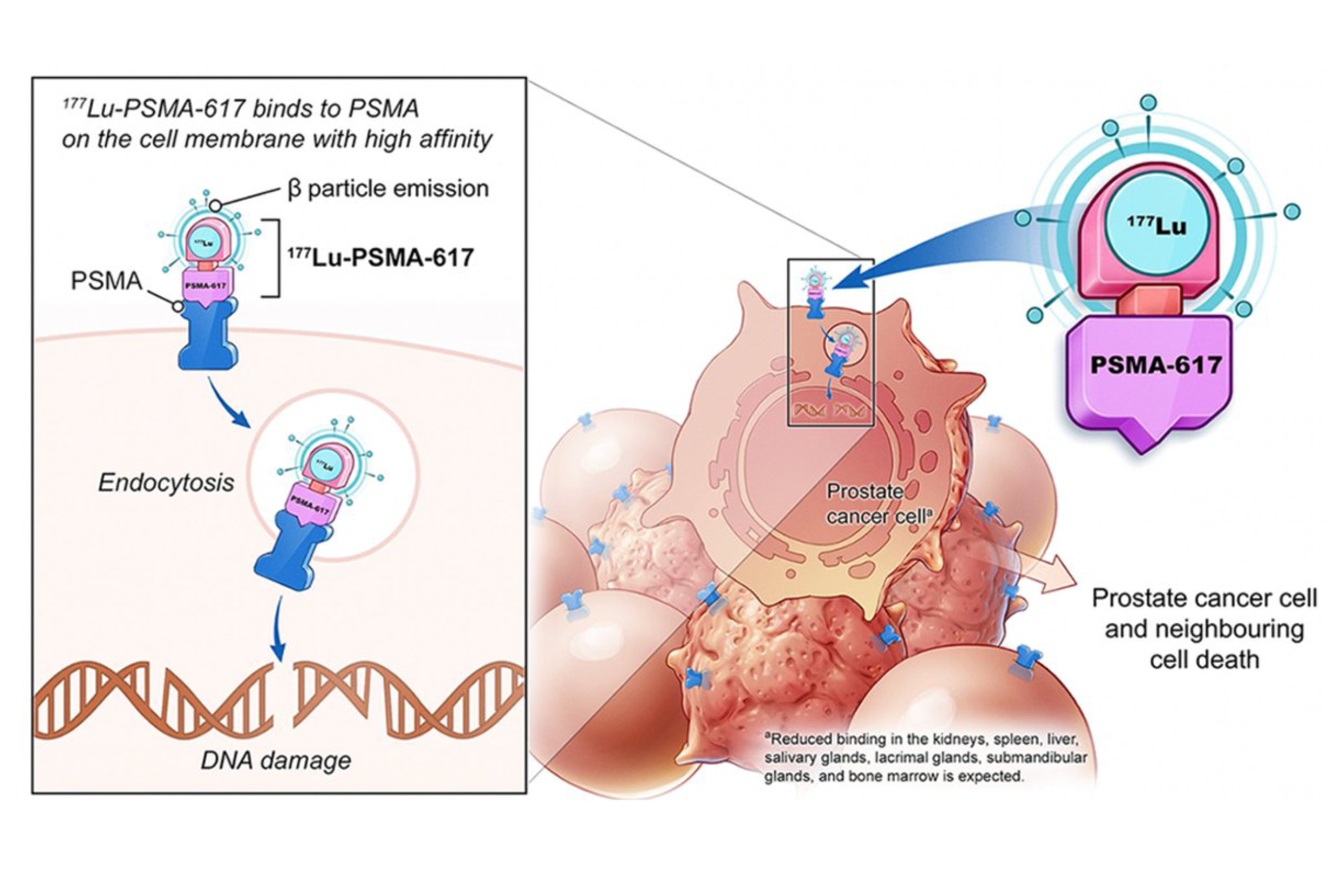
Astera Cancer Care is the first community cancer center in New Jersey to offer a novel treatment option for patients with metastatic prostate cancer. The novel therapeutic drug 177Lu-PSMA-617 is a prostate-specific membrane antigen (PSMA) targeting agent that is used to deliver radionuclide therapy for the treatment of patients with PSMA+ metastatic, castration-resistant prostate cancer (mCRPC).
Prostate cancer is the second leading cause of cancer death for men in the United States. Prostate cancer occurs due to the development and growth of abnormal cells in the prostate gland. mCRPC is an advanced form of prostate cancer that spreads beyond the prostate to other organs such as the lymph nodes, bones, bladder, rectum, and liver—the individual with mCRPC stops responding to hormone treatment known as androgen deprivation therapy (ADT).
Men with prostate cancer have several options for fighting the disease, including hormone therapy, chemotherapy, radiation, and surgery. However, if these treatments do not stop the progression of the cancer, there are few other options.
Pluvicto is administered once every six weeks for up to six doses. Pluvicto can significantly improve survival rates in individuals with progressing mCRPC. Astera has witnessed its success
Pluvicto, or 177Lu-PSMA-617, is the first FDA-approved targeted radioligand therapy for PSMA+ metastatic, castration-resistant prostate cancer. Formerly referred to as 177Lu-PSMA-617, the core component of Pluvicto is the no-carrier-added (nca) 177Lu (EndolucinBeta).
Results from the so-called VISION trial demonstrated that participants treated with Pluvicto, along with the best standard of care (BSoC), had a 38 percent reduction in risk of death and a 60 percent reduction in the risk of radiographic disease progression (rPFS) compared to BSoC alone. The most common adverse events (all grades) in the Pluvicto® part of the study were fatigue, dry mouth, nausea, anemia (low red blood cell counts), decreased appetite and constipation.
The results from the VISION study showed that Pluvicto plus BSoC, significantly improved overall survival in PSMA-positive mCRPC patients previously treated with AR pathway inhibition and taxane- based chemotherapy compared to BSoC alone.
Astera’s President, Dr. BrunoFang, led the VISION trial at Astera Cancer Care. He co-authored the VISION quantitative analysis abstract accepted at ASCO as an oral abstract presentation and the resulting The New England Journal of Medicine publication. Astera was among the top sites for enrolling subjects, and has witnessed its successes within its practice, which meant men with prostate cancer in New Jersey had the opportunity to access this drug as part of research long before the therapy’s FDA approval. Astera is a national leader in prostate cancer treatment and research, expanding access to clinical trials and the newest treatments.
Treatments, such as chemotherapy, can come with many unpleasant side effects. In contrast, Pluvicto has been proven to be a safe treatment for prostate cancer. The new drug targets PSMA, which is largely expressed on tumor cells. This can mean fewer side effects like nausea, vomiting and hair loss.
Pluvicto is a groundbreaking clinical advancement for prostate cancer patients and is a significant step forward in the evolution of precision radioligand therapy and demonstrates the importance of continued research and clinical trial participation, which is available at Astera.